Computer-aided key step generation in alkaloid total synthesis
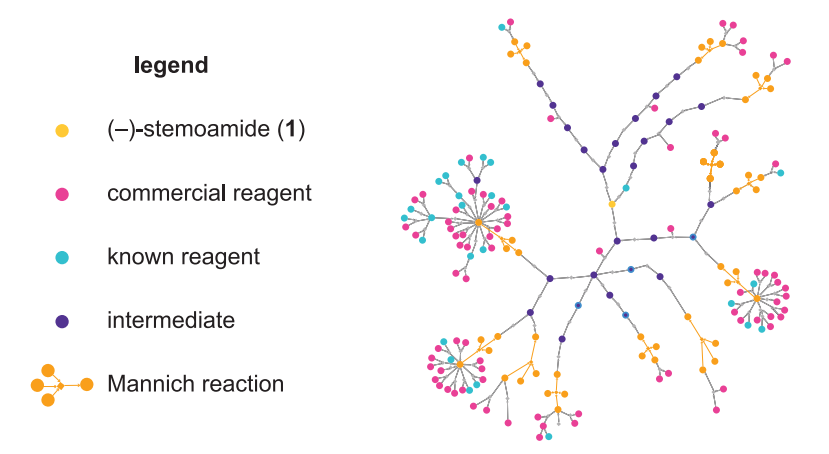
In this Science paper from 2023, researchers leveraged SYNTHIA™ Retrosynthesis Software to drastically reduce the number of steps required to synthesize stemoamide. From the abstract of the paper, “Efficient chemical synthesis is critical to satisfying future demands for medicines, materials, and agrochemicals. Retrosynthetic analysis of modestly complex molecules has been automated over the course of decades, but the combinatorial explosion of route possibilities has challenged computer hardware and software until only recently. Here, we explore a computational strategy that merges computer-aided synthesis planning with molecular graph editing to minimize the number of synthetic steps required to produce alkaloids. Our study culminated in an enantioselective three-step synthesis of (–)-stemoamide by leveraging high-impact key steps, which could be identified in computer-generated retrosynthesis plans using graph edit distances.”
Software that predicts synthetic routes to complex molecules has been rapidly increasing in sophistication. The extent to which the algorithms replicate or complement human intuition nonetheless remains uncertain. Lin et al. challenged a commercial retrosynthesis program with an alkaloid for which more than 30 syntheses had been reported in the literature. The algorithm consistently proposed a Mannich reaction that no prior approach had featured, although drawbacks in the rest of the route benefited from human intervention. By devising a supplemental graph-based method of prioritizing key steps, the authors achieved the shortest synthesis of this target. —JSY
To learn more, read the Full Paper Here.
