Synthesis of a Bridged Polycycle Sesquiterpene: Lamellodysidine A
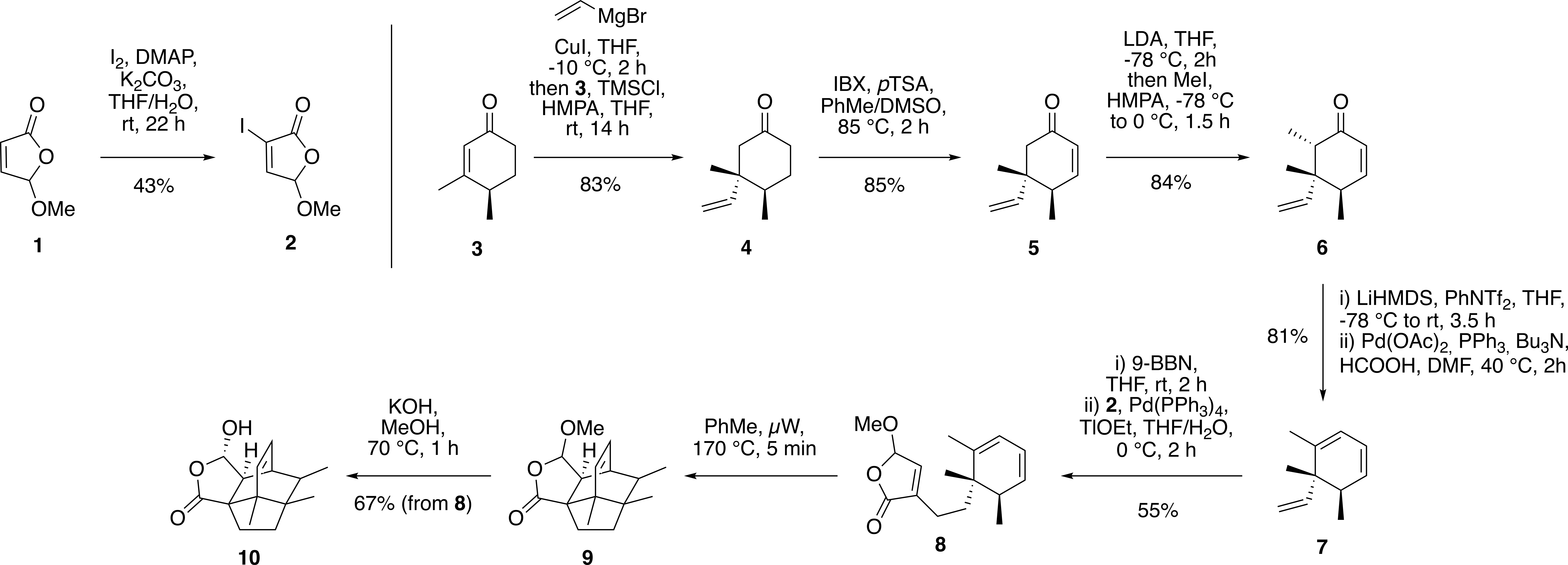
The synthesis of natural products in many cases is a challenging task due to the presence of complex 3D carbon scaffolds and a variety of functional groups. Lamellodysidine A is one of the recently reported bridged polycyclic sesquiterpenes with a unique carbon framework. This intriguing molecule was analyzed by SYNTHIA™ Retrosynthesis Software, which managed to identify a pathway with an intramolecular Diels-Alder reaction as a key step.
Notably, the software correctly predicted the stereochemistry of the Diels–Alder cycloadduct and the selective formation of the thermodynamically more stable and less hindered stereoisomer in the last step. The route was successfully executed in the laboratory and showed the utility of retrosynthetic software for planning syntheses even of challenging targets.
SYNTHIA™ Retrosynthesis Software can spark creativity in your synthetic planning by quickly designing routes for target molecules based on your requirements. Sign up for a live demo; contact us here.
