White paper:
Artificial-Intelligence-Driven Organic Synthesis— En Route towards Autonomous Synthesis?
Adapted from
C. Empel, R. M. Koenigs, Angew. Chem. Int. Ed. 2019, 58, 17114. https://doi.org/10.1002/anie.201911062
Published with the courtesy of Wiley.
Organic chemists’ creativity and meticulous discipline have enabled organic synthesis of complex molecules for decades and their synthesis is often referred to as “the art of synthesis.” Artificial intelligence algorithms are being developed and refined not only to perform literature searches and retrosynthetic analysis but also to identify and rank potential synthesis routes that include the reaction conditions. Embel and Koenigs review a recent Science article that combines artificial intelligence (AI)-driven development of synthetic plans for small molecules with an AI-developed automated synthesis process. They also discuss its limitations and highlight future innovations for enhancing the benefits of the AI-driven organic synthesis output.
Introduction
Traditionally, total synthesis of complex molecules including organic synthesis involves creativity, meticulous assessment of each step for yield of desired product, and reiterative process to modify the reactions for specific biochemical or biological properties (e.g., bioavailability, solubility) and optimize yields. AI is being used to propose reaction methodology such as novel pathways for synthesis of compounds. Empel and Koenigs suggest that the next evolutionary step in AI would be automated multistep synthesis of complex molecules. Although similar, automated and autonomous synthesis differ by their need for human input. Human input is required during automated synthesis to define thresholds, boundaries, reaction parameters, and synthesis protocols in the reaction files. In comparison, autonomous synthesis is a self-governing synthetic process that adjusts to surrounding parameters such as stereoselectivity and reaction yield without human input.
Limitations of Traditional Retrosynthetic Analysis and Automated Synthesis
Without AI support, chemists can easily miss pertinent combinations of novel substrates, reaction optimization, design of improved catalysts and novel reactions in the rapidly expanding body of chemical literature. All disciplines of chemical sciences consider synthesis of small molecules as a bottleneck and automated on-demand synthesis can help overcome this challenge.
Poorly soluble compounds provide extra challenges in automated workflows and often require more human input to improve the process because of clogging the channels. Predictions on the solubility of reactants in the proposed synthesis plan remain limited which hampers the execution Article 18 Green of some synthetic routes. Reactions that require or yield subambient temperatures usually also need advice from an expert chemist to maintain efficient workflow. After the automated synthesis, batch purification will be required for the final compound and may need special equipment such as particular columns.
AI-Planning of Synthesis Pathways and Automated Synthesis
Embel and Koenigs summarize the recent article by Jamison and Jensen and their colleagues [1]. They combined computer-aided retrosynthesis planning and a robotically reconfigurable flow apparatus to provide on-demand synthesis of small molecules (50-750 g/mole), as summarized in Figure 1.The system still requires human input tocomplement the AI synthetic algorithm withpractical considerations (e.g., precise stereochemistry and solvent choices) that help optimize the multistep synthesis process.
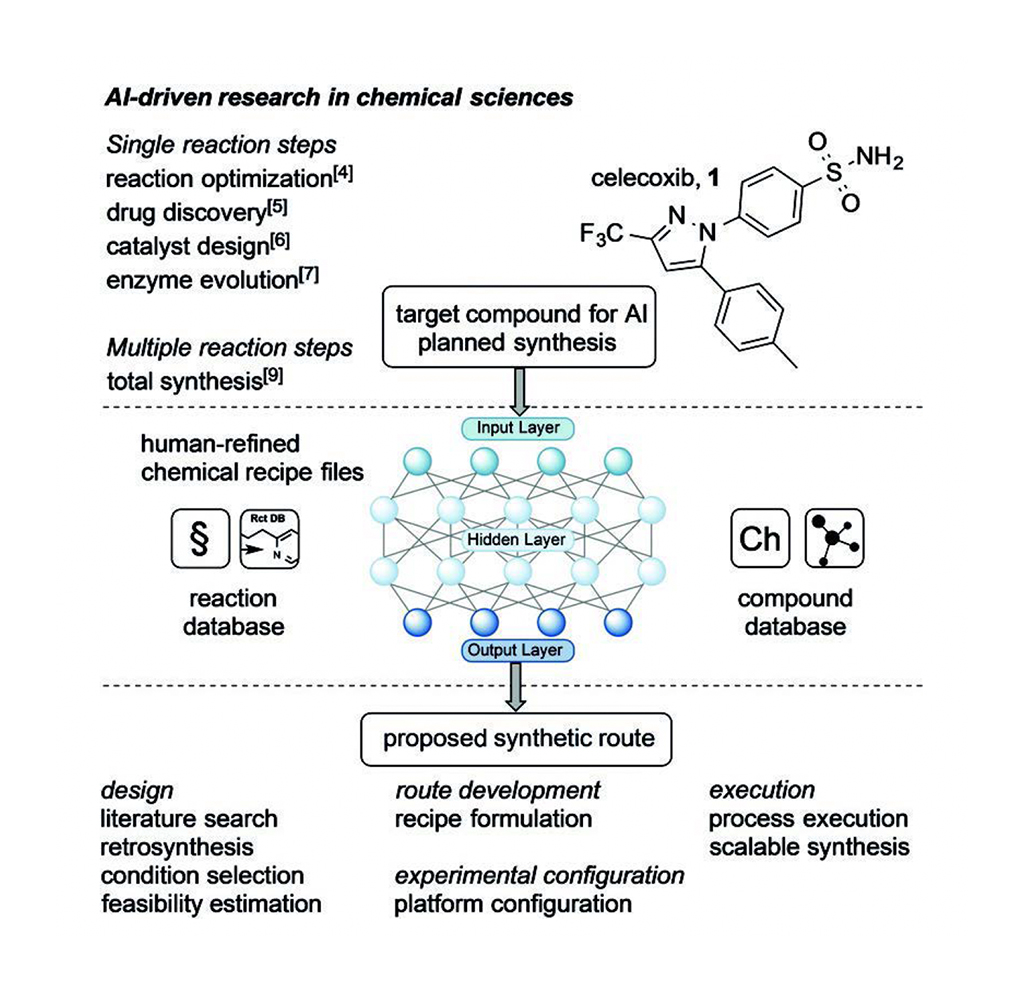
AI learns design principles from a literature search of databases that include retrosynthesis reactions and related compound reactions to design one or more synthetic routes. The proposed plans include reaction conditions, precursors, enzymes (as needed), catalysts, substrates, precursors, and by-products at each of the steps. AI also provides an estimation of the feasibility of each proposed synthetic plan so chemist(s) can choose the most appropriate plan for automation, often in microfluidics, with an intention to scale the process. After AI developed an in silico synthesis plan from the reaction and compound databases, chemists use their expert knowledge of synthesis to refine the chemical recipe files, and the experimental and platform configurations. Chemists adjust the recipe files to overcome any inadequacies of the microfluidic flow systems before it is used in a chemical bay in the automated system. Thus, the chemists’ input facilitates the robotic implementation of the proposed multistep synthesis, which is a major step toward scalable synthesis.
Jamison, Jensen and colleagues used this strategy to predict de novo synthesis routes of 15 small molecules, modify the relevant recipe files, and automate their synthesis using microfluidic workflows [1]. The 15 compounds include the nonsteroidal anti-inflammatory (NSAID) celecoxib, the blood thinner warfarin, and the ACE inhibitor prodrug, quinapril. Figure 2 (top panel) shows the synthetic process for the nonsteroidal anti-inflammatory (NSAID), celecoxib (structure 1). The first two robot-controlled reaction bays conducted the Claisen condensation of 4-methyl acetophenone (2) with methyl trifluoroacetate (3). Another robot-controlledreaction bay conducted the final condensation of the intermediate with hydrazine (4)and yielded celecoxib.
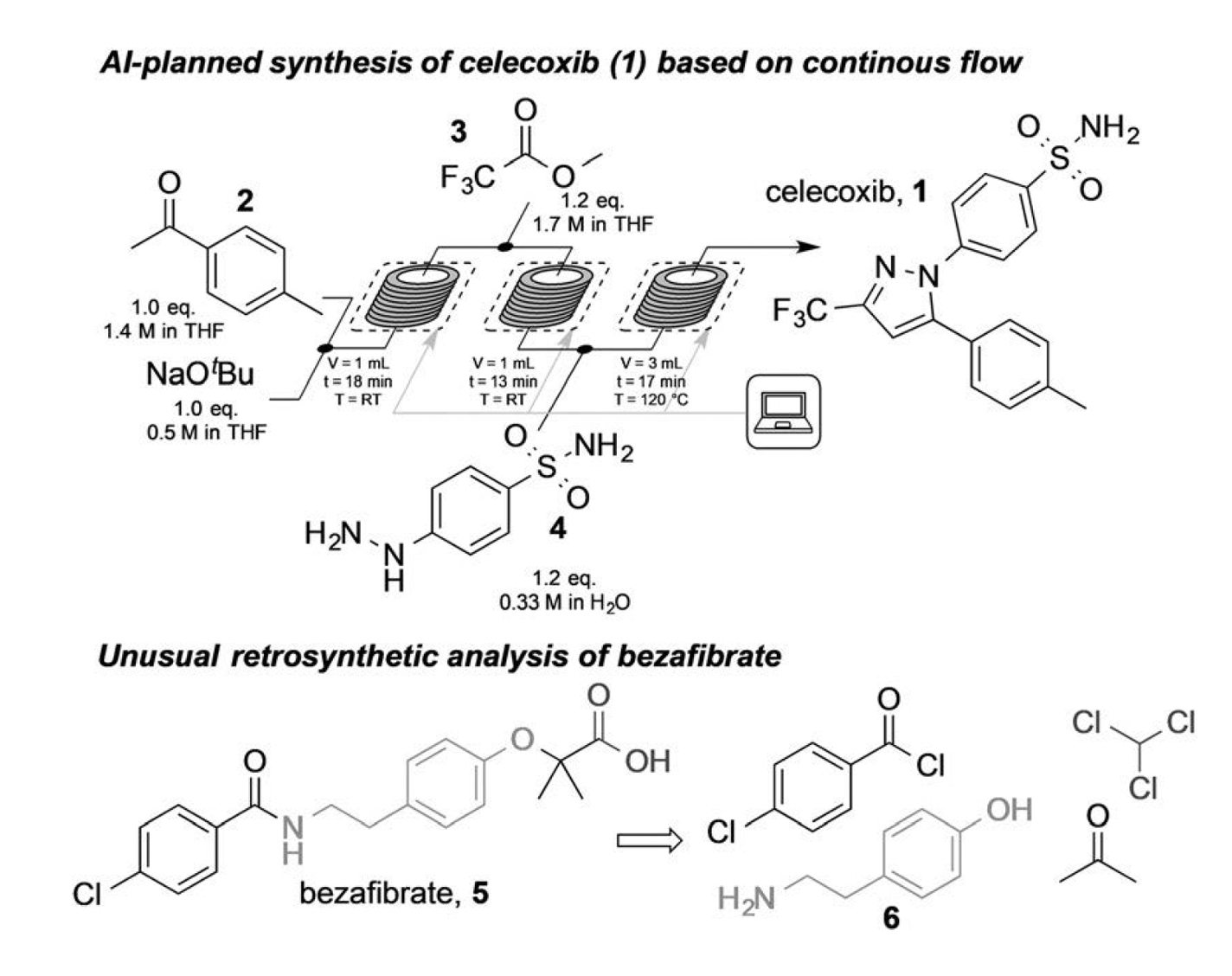
Jamison, Jensen and colleagues applied the AI-driven technology to develop synthesis plans and synthetic execution of multiple ACE inhibitors and numerous celecoxib analogues [1]. The yield of the small molecules ranged from 342 mg/h to 572 mg/h.
The AI proposed synthesis plan for bezafibrate used a Bargellini reaction involving acetone, chloroform, and phenol (6). However, subambient temperatures occurred during the attempted synthesis of bezafibrate (Fig. 2 compound 5) by microfluidics. Jamison, Jensen and colleagues demonstrated the feasibility of the AI proposed synthetic plan by performing a separate manual synthesis process. Its yield was 76%.
Future Improvements
Multiple groups are working on improving AI-driven analysis and prediction of stereochemistry and methods to favor the desired stereochemical structure in the proposed synthesis. This improvement could reduce the human input needed for the modification(s) and use of the chemical recipe files.
In the future, AI also may be able to propose continuous purification schemes or batch purification systems to provide relatively pure target compound. The addition of online reaction analytics could provide important information on reaction progress and allow feedback algorithms to alter reaction parameters in real time and potentially improve efficiency and yield.
Summary
Jamison, Jensen and colleagues described their AI-driven on-demand synthesis of small molecules (50-750 g/mole) by combining computer-aided retrosynthesis planning with chemist-refined reaction files that directed a robotically reconfigurable flow apparatus [1]. They used this strategy to predict de novo synthesis routes of 15 small molecules, modify the relevant recipe files, and automate their synthesis using microfluidic workflows. The 15 compounds include the nonsteroidal anti-inflammatory (NSAID) celecoxib, the blood thinner warfarin, and the ACE inhibitor prodrug, quinapril. Embel and Koenigs discussed limitations such as poor solubility of one or more components and target compounds requiring specific stereochemistry. They also suggested expanding the AI-driven proposed plans to include purification scheme(s) and /or in-process reaction analytics to further automate the synthesis process. These improvements would divert the routine synthesis and optimization work to robots so that chemists can spend more effort on curiosity-driven research projects, thorough reaction monitoring and analysis, and serendipitous discoveries.
References
[1] Coley, C.W. et al. (2019). A roboticplatform for flow synthesis of organic compounds informed by AI planning. Science.DOI: 10.1126/science.aax1566.
