White paper:
Synthetic Automations: A Revolution from Stone Age to Modern Era
Adapted from
Fang, G., Lin, D.-Z. and Liao, K. (2023), Synthetic Automations: A Revolution From “Stone Age” to Modern Era. Chin. J. Chem., 41: 1075-1079. https://doi.org/10.1002/cjoc.202200713
Published with the courtesy of Wiley.
Traditional organic synthesis has made outstanding progress but manual operation, inconsistent reproducibility, and inadequate efficiency hinder its dependable evolution to intelligent automation. Synthetic chemistry is beginning to embrace Artificial intelligence (AI) software to replace labor-intensive processes such as research for developing potential synthetic pathways, identifying reaction reagents with ranked choices of putative organic synthesis plans and automated synthesis. Herein, Liao and colleagues highlight some of the representative breakthroughs in automated synthesis and present current challenges and future directions in the field.
Background
The Industrial revolution in the 18th, and 19th centuries witnessed the power of automated manufacturing. Organic synthesis has depended on highly trained labor (chemists) to create and perform the molecular assembly process. In the 1960’s, Merrifield reported the first automated system in organic chemistry: solid-phase peptide synthesis by attaching the C-terminus to a resin and masking the N-terminus with a protective group. The automation set-up pumps in the relevant reagents and solvents, mixes them with the resin, and removes them in the correct order to achieve deprotection, acylation, separation, and purifications.
In most cases, organic synthesis remains a very time and labor consuming process that provides variable results due to differences in techniques in separate laboratories and facilities. In this Emerging Topic, Fang and colleagues highlight several recent breakthroughs such as AI-driven research, AI-assisted synthesis planning, and AI-integrated robot automation of the actual synthesis process. Challenges in design and implementation of the automated synthetic pathways are also presented.
Breakthroughs
Many drugs are small molecules with diverse chemical structures and thus, require customized procedures which consume both money and highly trained labor. Burke developed iterative carbon 2D and 3D cobalt catalysts (C-Csp2, C-Csp3) assembly strategy and automated the process to synthesize 14 diverse classes of small molecules. The use of tetramethyl N-methyliminodiacetic acid (TIDA) supported C-Csp3 bond formation. This synthesis machine coupled with more than 5000 commercial building blocks could support the synthesis of numerous small molecules.
Flow-based synthetic platforms can provide precise control of reaction temperatures, reaction times, and composition, and they can play major roles in automated synthesis. For example, the fast-flow device Tiny Tides invented by Li and Pentelute et al. could efficiently produce cell-penetrating peptides-conjugated peptide nucleic acid [1]. Mo et al. accelerated the discovery of novel electroorganic processes with high-throughput experimentation on their automated micro-fluidic single-droplet screening and analysis platform in 2020 [2]. In 2022, Wang et al. designed an electrocatalyst testing platform: It performed 942 effective tests on 109 copper-based bimetallic catalysts in 55 hr [3].
Gilmore and coworkers developed an automated multistep synthesizer that stably and reproducibly could provide both linear and convergent synthetic processes by arranging multiple continuous flow modules around a central core [4]. Switching from one module to another did not require manual configuration. The instrument also included inline monitoring with nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy: the monitoring facilitated post-reaction analysis and feedback. They demonstrated the flexibility of the radial flow configuration by synthesizing a library of derivatives of the anti-seizure drug, rufinamide.
Mo’s group described an automated platform that collected polarity estimations by inline thin-layer chromatography (TLC) in 2022 [5]. The trained AI-platform could estimate the probability of separation of multiple compounds and aid in proposing purification conditions.
To minimize the need for input from human chemists, Cronin’s group (2019) developed the Chemputer that provides methodological instructions into the individual steps and integrates the automation of the platform with bench-scale techniques using a natural language processing (NLP) algorithm [6]. The Chemputer system could extract synthetic procedures from publications, transform the synthesis plan into chemical description language used for procedures, convert the instructions into commands for manipulation of the automated platform, and direct the chemical synthesis. Without human intervention, the Chemputer assembled three high-quality pharmaceuticals with higher yields and purities than those achieved during manual synthetic and purification procedures A milestone in automation of organic chemical synthesis was accomplished by Coley et al. [7]. Their computer-aided chemical synthesis program involved synthetic planning based on millions of published chemical reactions and in silico simulations to maximize success. The AI-synthesis program directed a modular continuous flow platform that executed the synthesis by automatically reconfiguring the robotic arm. Its power was demonstrated by planning and synthesizing 15 compounds, including several angiotensin-converting enzyme (ACE) inhibitors and NSAID drugs.
Grzybowski and Burke et al. described an iterative machine learning system to explore general reaction conditions in the proposed automated synthesis protocol [8]. A simple closed-loop workflow leveraged the machine-learned data-guided matrix to prioritize and select subsequent reactions for testing, and used robotic experimentation to augment precision, throughput, and reproducibility. Its workflow through experimentation and machine learning identified reaction conditions for the hetero(aryl Suzuki-Miyaura coupling reaction which confirmed its utility for multidimensional chemical optimization difficulties.
Cooper’s group described the first AI-integrated mobile robot that autonomously ran 688 reactions over eight days to thoroughly test ten variables experimentally [9]. However, the robot did not have the software capacity to capture the existing chemical knowledge, nor machine learning for generating novel scientific hypotheses.
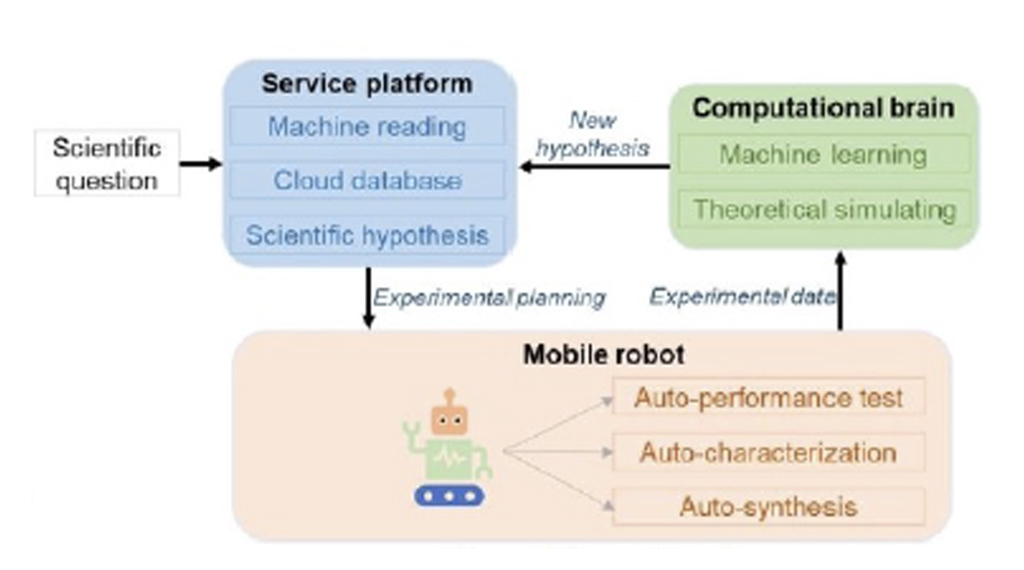
Jiang’s group (2022) described an AI-platform, called AI-Chemist, that could perform the essential steps for proposing and ranking synthetic planning, execution of the synthetic steps, monitoring and data collection of the synthesis process through multiple reactions and steps, and machine learning (Fig. 1) [10].
Challenge and Outlook
Much progress in automatic and autonomous AI-driven platforms for organic synthesis have been accomplished. However, widespread adoption would be hastened by addressing the following challenges.
Seamless integration of the automated synthetic platform which contains modules for reagent storage, reaction preparation module, numerous reactor modules, integrated analytical instrument(s) for monitoring reactions, purification system, management system for compound, monitoring unit, and console. Due to space limitations in most laboratories, the size of the instruments and platform ideally would be no larger than a fume hood. The algorithm(s) for computer-aided or AI-driven synthesis planning should be integrated with the computer-aided chemical synthesis (CASP) platform and the monitoring equipment seamlessly.
The physical platform and the software should be easily customizable and reconfigured for future uses.
The full unit should be reasonably (low) priced since many laboratories would hire actual chemists rather than the automation units at similar costs.
The automation platform and software need to be user-friendly: straight-forward in set up and optimization of synthetic planning/ ranking and selection and execution of the synthetic process. Using a universal chemical program language for the chemists’ input and retrieval of data will help maximize its benefit to human chemists.
As use of automation of the organic chemical synthesis expand, organic chemists will be relieved of repetitive experimentation often used during optimization. Organics chemists will be able to focus more time on answering the questions, “What should we synthesis? Why?” rather than the mechanics of the actual synthesis.
Summary
Multiple groups are advancing AI-driven organic chemistry synthesis, coupling machine learning to propose and experimentally test promising novel synthetic routes automatically with variable needs for human input. Automated synthesis can improve higher yields reliably and liberate chemists from routine manual tasks so they can focus on creative tasks.
References
[1] Li, C. et al. (2022). Automated Flow Synthesis of Peptide-PNA Conjugates. ACS Central Science. DOI: 10.1021/acscentsci.1c01019.
[2] Mo, Y. et al. (2020). A Multifunctional Microfluidic Platform for High-Throughput Experimentation of Electroorganic Chemistry. Angewandte Chemie - International Edition. DOI: 10.1002/ anie.202009819.
[3] Xie, M. et al. (2022). Fast Screening for Copper-Based Bimetallic Electrocatalysts: Efficient Electrocatalytic Reduction of CO2 to C2+ Products on Magnesium-Modified Copper. Angewandte Chemie - International Edition. DOI: 10.1002/anie.202213423.
[4] Chatterjee, S. et al. (2020). Automated radial synthesis of organic molecules. Nature. DOI: 10.1038/s41586-020- 2083-5.
[5] Xu, H. et al. (2022). High-throughput discovery of chemical structure-polarity relationships combining automation and machine-learning techniques. Chem. DOI: 10.1016/j.chempr.2022.08.008.
[6] Steiner, S. et al. (2019). Organic synthesis in a modular robotic system driven by a chemical programming language. Science. DOI: 10.1126/science.aav2211.
[7] Coley, C.W. et al. (2019). A robotic platform for flow synthesis of organic compounds informed by AI planning. Science. DOI: 10.1126/science.aax1566.
[8] Angello, N.H. et al. (2022). Closed-loop optimization of general reaction conditions for heteroaryl Suzuki-Miyaura coupling. Science. DOI: 10.1126/science. adc8743.
[9] Burger, B. et al. (2020). A mobile robotic chemist. Nature. DOI: 10.1038/s41586- 020-2442-2.
10] Zhu, Q. et al. (2022). An all-round AI-Chemist with a scientific mind. National Science Review. DOI: 10.1093/ nsr/nwac190.
